Made in the United States
When appropriate patients need ELUCIREM, you can depend on Guerbet
We are committed to making ELUCIREM accessible for pharmacy buyers so doctors can schedule diagnostic scans with confidence.
We’ve mastered the gadopiclenol manufacturing process through our history of macrocyclic GBCA innovation.
We made gadopiclenol commercially available in the first quarter of 2023.
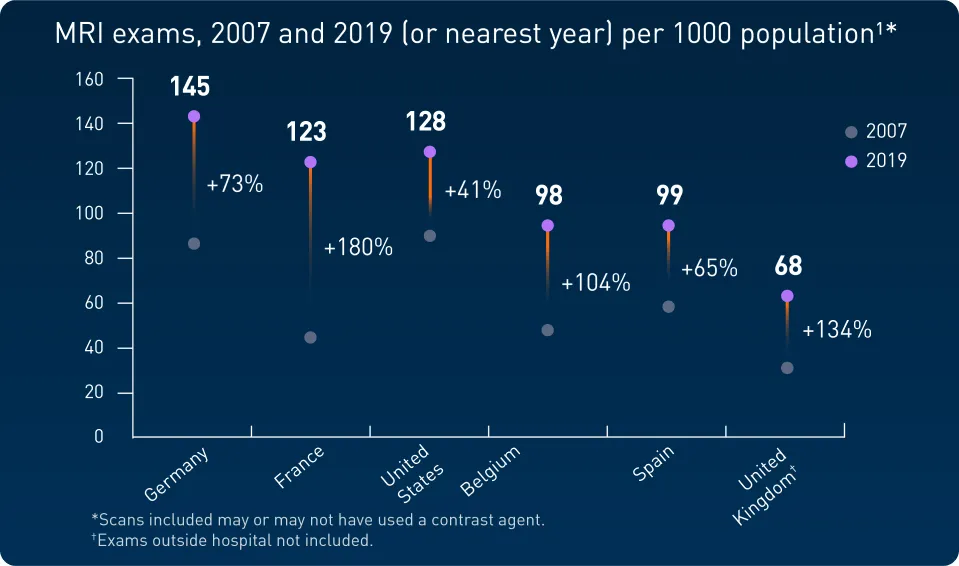
The consumption of GBCAs has increased1-3
Over 30 million doses of GBCA are consumed worldwide thanks to higher rates of MRI use as of 20141-3
Major health and medical authorities recommend minimizing gadolinium-based imaging in high-risk patients4-6
In response to reports of nephrogenic systemic fibrosis (NSF) and the retention characteristics of individual agents like gadolinium, the US Food & Drug Administration (FDA) and American College of Radiology (ACR) call for greater consideration when preparing MRI patients for body or CNS scans4-6
References
1. https://www.oecd-ilibrary.org/sites/eadc0d9den/index.html?itemId=/content/component/eadc0d9d-en.
Accessed September 2022. 2. OECD iLibrary. Diagnostic technologies. https://www.oecd-ilibrary.org/sites/ae3016b9-en/1/3/5/9/index.html?itemId=/content/publication/ae3016b9-en&_csp_=ca413da5d44587bc56446341952c275e&itemIGO=oecd&itemContentType=book. Accessed September 2022. 3. VM Runge. Safety of the gadolinium-based contrast agents for magnetic resonance imaging, focusing in part on their accumulation in the brain and especially the dentate nucleus. Invest Radiol. 2016;51(5):273-279. 4. FDA Drug Safety Communication: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warnings. US Food & Drug Administration; 2018. 5. ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media. April 2022. 6. Lancelot E, Desché P. Gadolinium retention as a safety signal: experience of a manufacturer. Invest Radiol. 2020;55(1):20-24.
When appropriate patients need ELUCIREM, you can depend on Guerbet
We are committed to making ELUCIREM accessible for pharmacy buyers so doctors can schedule diagnostic scans with confidence.
We’ve mastered the gadopiclenol manufacturing process through our history of macrocyclic GBCA innovation.
We made gadopiclenol commercially available in the first quarter of 2023.
TEST VIDEO
GU03250043
ELUCIREMTM (gadopiclenol) injection Important Safety Information
WARNING: RISK ASSOCIATED WITH INTRATHECAL USE and NEPHROGENIC SYSTEMIC FIBROSIS (NSF) Risk Associated with Intrathecal Use ELUCIREM is not approved for intrathecal use. Nephrogenic Systemic Fibrosis The risk for NSF appears highest among patients with: o Chronic, severe kidney disease (GFR <30 mL/min/1.73 m2), or o Acute kidney injury. Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (for example, age >60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing. For patients at highest risk for NSF, do not exceed the recommended ELUCIREM dose and allow a sufficient period of time for elimination of the drug from the body prior to any re-administration. |
Indications and Usage
ELUCIREMTM (gadopiclenol) injection is indicated in adult and pediatric patients aged 2 years and older for use with magnetic resonance imaging (MRI) to detect and visualize lesions with abnormal vascularity in the central nervous system (brain, spine, and associated tissues), and the body (head and neck, thorax, abdomen, pelvis, and musculoskeletal system).
Contraindications
Contraindicated in patients with history of hypersensitivity reactions to ELUCIREM.
Warnings and Precautions
- Risk Associated with Intrathecal Use: Intrathecal administration of GBCAs can cause serious adverse reactions including death, coma, encephalopathy, and seizures. The safety and effectiveness of ELUCIREM have not been established with intrathecal use. ELUCIREM is not approved for intrathecal use.
- Nephrogenic Systemic Fibrosis: GBCAs increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of ELUCIREM among these patients unless the diagnostic information is essential and not available with non-contrast MRI or other modalities.
- Hypersensitivity Reactions: With GBCAs, serious hypersensitivity reactions have occurred. Before ELUCIREM administration, assess all patients for any history of a reaction to contrast media, bronchial asthma and/or allergic disorders. These patients may have an increased risk for a hypersensitivity reaction to ELUCIREM.
- Gadolinium Retention: Gadolinium is retained for months or years in several organs. The highest concentrations have been identified in the bone, followed by other organs (e.g. brain, skin, kidney, liver, and spleen). While clinical consequences of gadolinium retention have not been established in patients with normal renal function, certain patients might be at higher risk. These include patients requiring multiple lifetime doses, pregnant and pediatric patients, and patients with inflammatory conditions. Minimize repetitive GBCA imaging studies, particularly closely spaced studies when possible.
- Acute Kidney Injury: In patients with chronically reduced renal function, acute kidney injury requiring dialysis has occurred with the use of GBCAs. The risk of acute kidney injury may increase with increasing dose of the contrast agent.
- Extravasation and Injection Site Reactions: Injection site reactions such as injection site pain have been reported in the clinical studies with ELUCIREM. Extravasation during ELUCIREM administration may result in tissue irritation. Ensure catheter and venous patency before the injection of ELUCIREM.
- Interference with Visualization of Lesions Visible with Non-Contrast MRI: As with any GBCA, ELUCIREM may impair the visualization of lesions seen on non-contrast MRI.
Adverse Reactions:
In clinical trials, the most frequent adverse reactions that occurred in > 0.2% of patients who received ELUCIREM included: injection site pain, headache, nausea, injection site warmth, injection site coldness, dizziness, and localized swelling.
Postmarketing Experience with use of ELUCIREM or other GBCAs: Acute pancreatitis, acute respiratory distress syndrome, pulmonary edema.
Use in Specific Populations
- Pregnancy: GBCAs cross the human placenta and result in fetal exposure and gadolinium retention. There are no available data on ELUCIREM use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes.
- Lactation: There are no data on the presence of ELUCIREM in human milk, the effects on the breastfed infant, or the effects on milk production. However, published lactation data on other GBCAs indicate that 0.01 to 0.04% of the maternal gadolinium dose is excreted in breast milk.
- Pediatric Use: The safety and effectiveness of ELUCIREM have not been established in pediatric patients younger than 2 years of age.
- Geriatric Use: This drug is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function.
- Renal Impairment: In patients with renal impairment, the exposure of gadopiclenol is increased compared to patients with normal renal function. This may increase the risk of adverse reactions such as NSF. No dose adjustment of ELUCIREM is recommended for patients with renal impairment.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see the full Prescribing Information, including the Medication Guide, for additional important safety information.